 Adequate bone volume is widely agreed to be the primary pre-requisite for achieving a positive long-term prognosis for dental implant treatment.[i] Guided bone regeneration (GBR) has been described as the gold standard for regenerating bone in an atrophic ridge.[ii] As part of this treatment, a customised titanium mesh is often used to increase bone volume in areas of significant bone deficiency. The predictability of this approach is assisted by an awareness of developments in mesh design and materials, as well as attention to the processes that enable bone healing and regeneration during GBR.
Adequate bone volume is widely agreed to be the primary pre-requisite for achieving a positive long-term prognosis for dental implant treatment.[i] Guided bone regeneration (GBR) has been described as the gold standard for regenerating bone in an atrophic ridge.[ii] As part of this treatment, a customised titanium mesh is often used to increase bone volume in areas of significant bone deficiency. The predictability of this approach is assisted by an awareness of developments in mesh design and materials, as well as attention to the processes that enable bone healing and regeneration during GBR.
What is GBR?
GBR was introduced in 1988, after studies suggested that bone regeneration is significantly enhanced when soft tissue is mechanically prevented from invading osseous defects.[iii] GBR aims to facilitate bone growth by providing a protective layer over regenerative material, isolating the bony defect from connective tissue. The mesh used in this process also acts as a scaffold to support new growth.[iv]
For GBR to be successful, four key principles have to be achieved. There has to be effective closure of the wound to establish uninterrupted healing. Epithelium and connective tissue have to be excluded without interfering with angiogenesis to provide enough blood supply. There has to be adequate structure to provide suitable space for bone regeneration. Finally, the fibrin clot and implanted material must remain stable to prevent interruption to the healing process.[v]
Summarising the GBR barrier materials
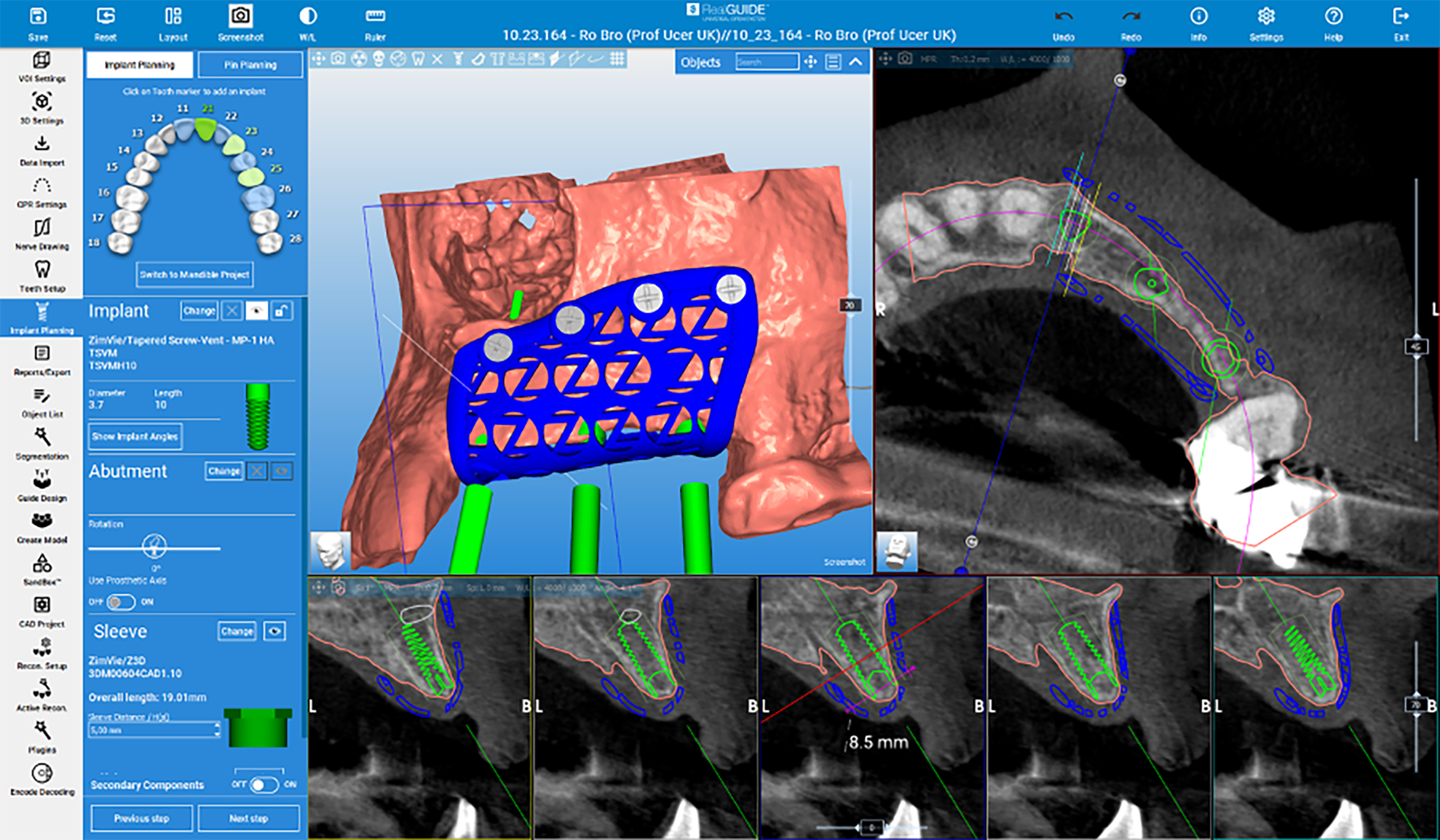
Barrier materials used in guided bone regeneration can either be resorbable or non-resorbable, and in recent years many different materials have been tested. Non-resorbable materials such as titanium foils, perforated titanium meshes, titanium-reinforced polytetrafluoroethylene (PTFE) can be pre-shaped or customised.[vi]
Expanded PTFE is a chemically stable and biologically inert polymer with a porous structure and flexible form. Stabilised with pins and screws, PTFE membranes have been considered the most predictable tools for increasing bone volume in large alveolar ridge deficiencies before or during implant surgery.[vii] However, some studies have found their advantages over pure titanium to be minimal.[viii] One of the main disadvantages of non-resorbable barriers is the higher risk of soft tissue exposure and associated complications including graft failure and infections.
Non-resorbable membranes require a second surgical intervention for their removal. The second surgery also adds to the patient’s costs, chair and recovery time.[ix] However, non-resorbable titanium meshes are highly effective in maintaining the desired shape between the barrier and bone defect. Pores in the design of titanium meshes also allow vascularisation in soft tissue and bone to be maintained. In vitro tests have shown that titanium membranes with larger pores result in greater bone growth than microporous and resorbable membranes.[x]
Resorbable membranes were developed to reduce the number of surgical procedures required in the GBR process, as they allow the implanted membrane to be gradually replaced by the patient’s natural tissue. Several resorbable membranes have been tested showing various degrees of successful bone regeneration. Natural sources include collagen, gelatine, alginate, chitosan and silk fibroin, and synthetic sources include polyglycolic acid (PGA), polycaprolactone (PCL), polyethylene glycol (PEG) or poly(ethylene oxide) (PEO), and their copolymers.[xi]
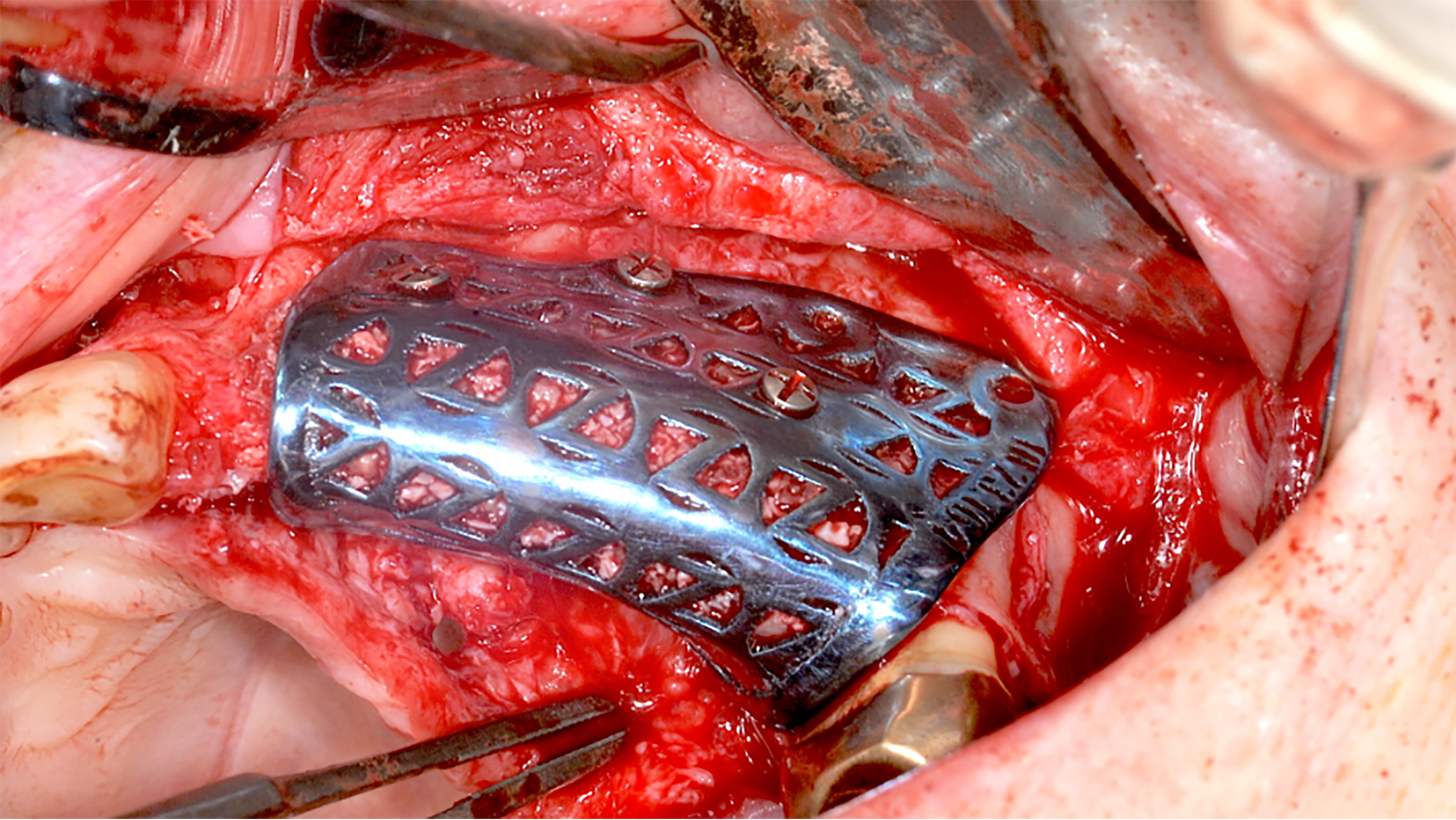
In comparison to titanium and PTFE membranes, resorbable meshes have poor mechanical stability and can only maintain barrier functionality for a limited time before being resorbed, which currently restricts their real-world application particularly in vertical augmentation cases.[xii]
Patient specific customised 3D alveolar bone reconstruction
Patient-specific customised 3D manufactured titanium mesh is an innovative technique for alveolar bone regeneration, offering a highly precise and tailored approach to bone reconstruction. Leveraging advancements in digital workflows and CAD/CAM technology, this method enables the design and 3D printing of individualized barrier devices that conform precisely to the patient’s defect morphology.
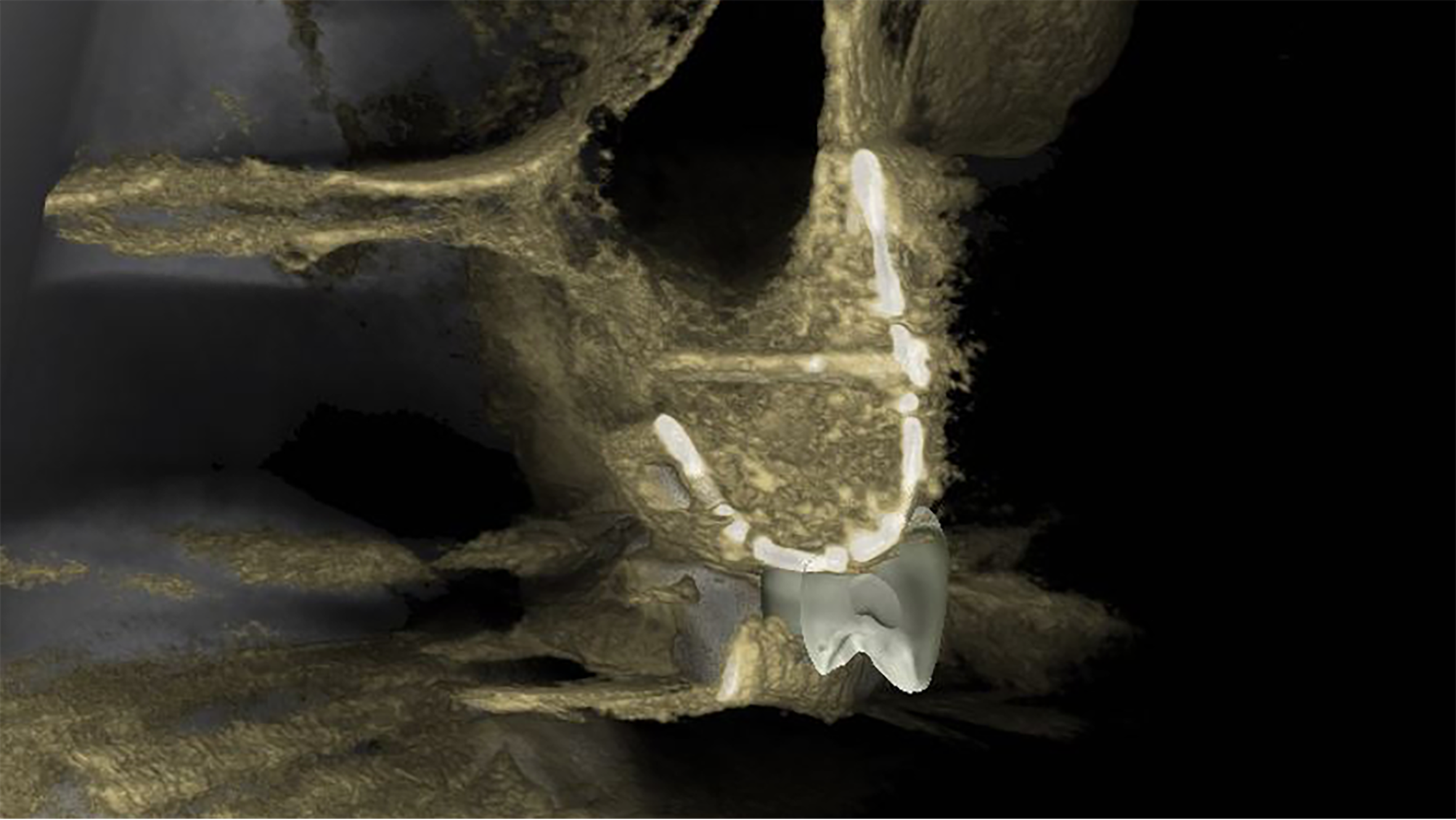
A cross sectional CBCT view showing the vertical bone regeneration
These titanium meshes are produced through additive manufacturing and provide rigid yet biocompatible scaffolding to support bone regeneration while maintaining space for graft materials. The custom-fit design simplifies complex surgical procedures, reduces intraoperative adjustments, and improves outcomes by minimizing complications and post-operative discomfort. A similar technique involves custom design and manufacture of block bone grafts specifically to fit the individual patient’s defects. These grafts are 3D designed and milled from of donated bone allografts which fit with precision and become remodelled into living bone tissue for future implant placements.
Hands-on experience
Achieving predictable and effective reconstruction of large alveolar bone defects forms part of the hands-on cadaveric surgical training in a special course run by eminent specialist oral surgeon, Professor Cemal Ucer. Delivered in partnership with the University of Salford, Manchester and ICE Postgraduate Dental Institute and Hospital, the Advanced Certificate In Bone & Tissue Regeneration & Sinus Grafting, offers participants comprehensive, evidence-based insight and training in regenerative oral surgery and implantology.
As the gold standard in predictably creating adequate bone volume for implant surgery, GBR and other grafting solutions offer edentulous patients with severe bone defects hope in achieving successful treatment outcomes. Maintaining an awareness of the most effective materials and methods as clinicians develop an advanced practice enables them to offer their patients this hope with greater confidence.
Please contact Professor Ucer at ucer@icedental.institute or Mel Hay at mel@mdic.co
01612 371842
[i] Aceves-Argemí R, Roca-Millan E, González-Navarro B, Marí-Roig A, Velasco-Ortega E, López-López J. Titanium Meshes in Guided Bone Regeneration: A Systematic Review. Coatings. 2021; 11(3):316. https://doi.org/10.3390/coatings11030316
[ii] Alazmi SO. A review on guided bone regeneration using titanium mesh. Bioinformation. 2024 May 31;20(5):562-565. doi: 10.6026/973206300200562. PMID: 39132237; PMCID: PMC11309112.
[iii] Saad M, Assaf Am Maghaireh H. Guided Bone Regeneration: Evidence & Limits. Smile Dental Journal | Volume 7, Issue 1 – 2012
[iv] De Santis D, Gelpi F, Verlato G, Luciano U, Torroni L, Antonucci N, Bernardello F, Zarantonello M, Nocini PF. Digital Customized Titanium Mesh for Bone Regeneration of Vertical, Horizontal and Combined Defects: A Case Series. Medicina (Kaunas). 2021 Jan 11;57(1):60. doi: 10.3390/medicina57010060. PMID: 33440889; PMCID: PMC7826518.
[v] Abtahi S, Chen X, Shahabi S, Nasiri N. Resorbable Membranes for Guided Bone Regeneration: Critical Features, Potentials, and Limitations. ACS Mater Au. 2023 Jun 23;3(5):394-417. doi: 10.1021/acsmaterialsau.3c00013. PMID: 38089090; PMCID: PMC10510521.
[vi] Aceves-Argemí R, Roca-Millan E, González-Navarro B, Marí-Roig A, Velasco-Ortega E, López-López J. Titanium Meshes in Guided Bone Regeneration: A Systematic Review. Coatings. 2021; 11(3):316. https://doi.org/10.3390/coatings11030316
[vii] De Santis D, Gelpi F, Verlato G, Luciano U, Torroni L, Antonucci N, Bernardello F, Zarantonello M, Nocini PF. Digital Customized Titanium Mesh for Bone Regeneration of Vertical, Horizontal and Combined Defects: A Case Series. Medicina (Kaunas). 2021 Jan 11;57(1):60. doi: 10.3390/medicina57010060. PMID: 33440889; PMCID: PMC7826518.
[viii] Aceves-Argemí R, Roca-Millan E, González-Navarro B, Marí-Roig A, Velasco-Ortega E, López-López J. Titanium Meshes in Guided Bone Regeneration: A Systematic Review. Coatings. 2021; 11(3):316. https://doi.org/10.3390/coatings11030316
[ix] Briguglio, F., Falcomatà, D., Marconcini, S., Fiorillo, L., Briguglio, R., Farronato, D., The Use of Titanium Mesh in Guided Bone Regeneration: A Systematic Review, International Journal of Dentistry, 2019, 9065423, 8 pages, 2019. https://doi.org/10.1155/2019/9065423
[x] Briguglio, F., Falcomatà, D., Marconcini, S., Fiorillo, L., Briguglio, R., Farronato, D., The Use of Titanium Mesh in Guided Bone Regeneration: A Systematic Review, International Journal of Dentistry, 2019, 9065423, 8 pages, 2019. https://doi.org/10.1155/2019/9065423
[xi] Abtahi S, Chen X, Shahabi S, Nasiri N. Resorbable Membranes for Guided Bone Regeneration: Critical Features, Potentials, and Limitations. ACS Mater Au. 2023 Jun 23;3(5):394-417. doi: 10.1021/acsmaterialsau.3c00013. PMID: 38089090; PMCID: PMC10510521.
[xii] Abtahi S, Chen X, Shahabi S, Nasiri N. Resorbable Membranes for Guided Bone Regeneration: Critical Features, Potentials, and Limitations. ACS Mater Au. 2023 Jun 23;3(5):394-417. doi: 10.1021/acsmaterialsau.3c00013. PMID: 38089090; PMCID: PMC10510521.












